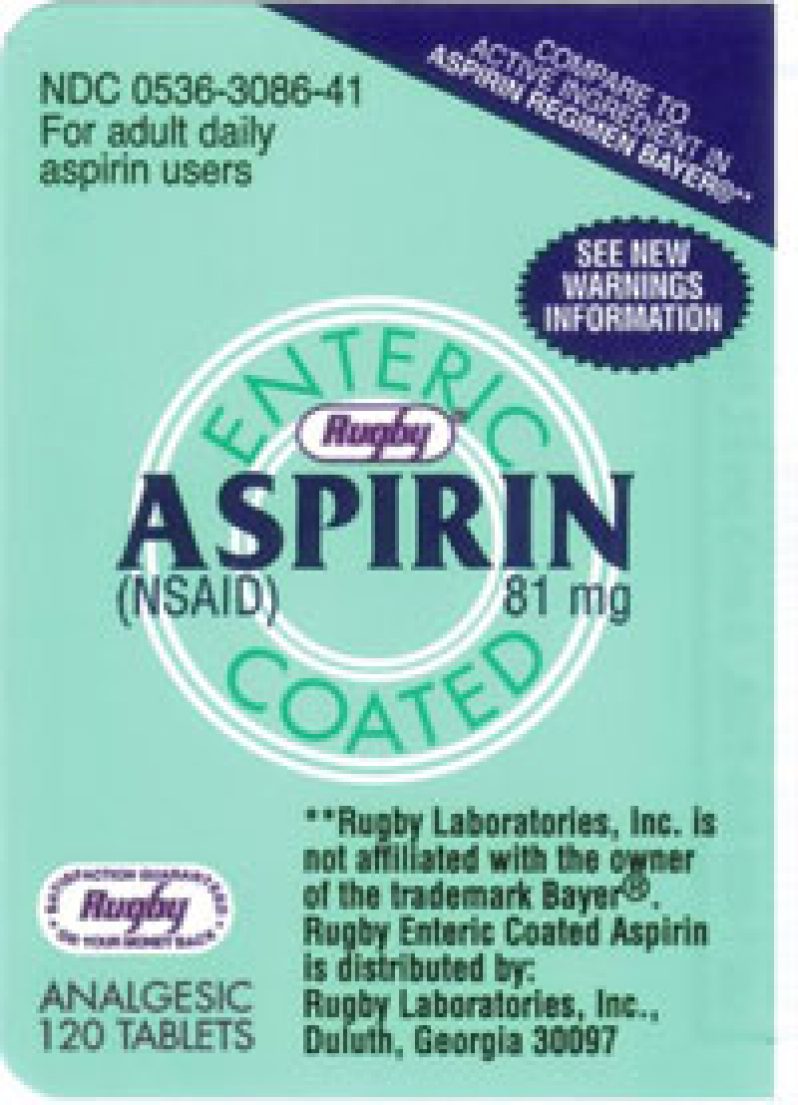
A LABELLING mix-up has prompted the Government Analyst at the Food and Drug Department to announce a recall of ‘Rugby’ enteric-coated aspirin tablets.
 The recall applies to the 81 mg ‘Rugby’ enteric-coated tablets from batch number 13A026. The pills which the Food and Drug Department says have a January 2015 expiry date, are manufactured and packaged by Advance Pharmaceutical Inc. under the label, ‘Rugby Laboratories (Major Pharmaceuticals)’.
The recall applies to the 81 mg ‘Rugby’ enteric-coated tablets from batch number 13A026. The pills which the Food and Drug Department says have a January 2015 expiry date, are manufactured and packaged by Advance Pharmaceutical Inc. under the label, ‘Rugby Laboratories (Major Pharmaceuticals)’.
“Importers, distributors, retailers and consumers who are in possession of this lot of aspirin are asked to stop distributing, selling or using this product and inform the department,” the Food and Drug Department said in a press release.
Bottles of pills which were labelled as containing 81 mg aspirin pills actually contained 500 mg acetaminophen tablets.
“Customers may be inadvertently taking acetaminophen 500 mg tabs instead of enteric-coated Aspirin 81 mg [tabs],” the release warns. Consumers of the wrongly labelled pills may experience an overdose of acetaminophen if they also happen to be taking other medication containing it (acetaminophine).
An acetaminophen overdose may cause severe liver damage, the release stated.
The Food and Drug Department, located in the Institute of Applied Science and Technology (IAST) Building on the Turkeyen campus of the University of Guyana, can be contacted on telephone number 222-8857, via fax: 222-8856 or through its email address at, foodanddrug@health.gov.gy .



.jpg)