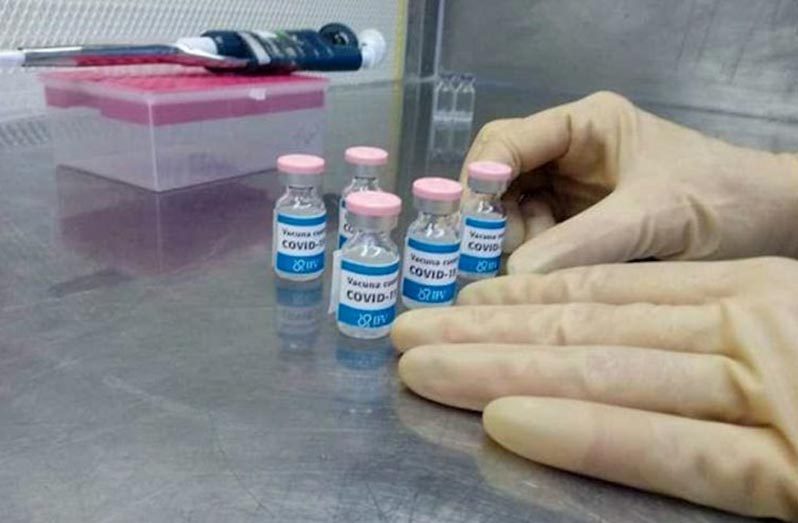
CUBA, on Monday, commenced a vaccine trial to fight COVID-19 which could save millions of lives globally, according to President of Guyana-Cuba Solidarity Movement (GCSM), Halim Khan.
The trial is expected to conclude on January 11, 2021. He explained that the Center for State Control of Medicines, Equipment and Medical Devices (CECMED) approved studies developed by the Finlay Vaccine Institute.
The research to develop the vaccine, he said, covers 676 people between the ages of 19 and 80 and it will be randomized, controlled, adaptive and multi-centred.
He explained that the study will be a “randomized controlled, double-blind trial,” meaning doctors and participants will not know who will be injected with the vaccine candidate.
Recipients, he said, will be selected randomly. Those in the control group will get another vaccine produced in Cuba.
“Its aim is to assess the safety, reactogenicity and immunogenicity of the vaccine candidate in a two-dose scheme,” Khan added.
The GCSM president noted that the results of that trial would be available on February 1 to be published on February 15.
He noted that Cuban President, Miguel Diaz-Canel, confirmed the progress of the Cuban project and showed confidence in the response to prevent the spread of the novel coronavirus.